
Conform Clinical Development
Smarter Pathways from Concept to Cure.
Who We Are
Conform Clinical Development (CCD) is a research organization focused on strategic product development for biotech and pharmaceutical companies.We’ve pioneered a resourceful model that reduces overhead costs, ensures milestone delivery, and accelerates time-to-market.
We revolutionize biotech and pharma development with strategy, science, and speed.

Our Approach
We have created an intellectual paradigm supported by the foundation of an optimized project management process, a regulatory compliance management system, and an assortment of strategies to achieve significant cost and time efficiencies.This proven strategy empowers our expert drug development navigators, business development strategists, and subject matter experts (SME) to focus on the critical path that facilitates deployment for proficient planning of drug development programs.
Reduced Overhead
Lean operations that cut unnecessary costs without compromising quality.
Milestone-Driven Delivery
Strategic planning that aligns with clear, trackable goals.
Risk-Aware Commercialization
Minimizing risk with proactive exit strategies at every phase.

We are scientists, strategists, and innovators on a mission!
Our Model:
Reverse-Engineered for Success
🔄 Reverse Engineering ApproachStarting from the end milestone, we reverse-engineer the full research pathway—customized, efficient, and exit-focused.🧠 Intellectual ParadigmProven strategy supported by project management, regulatory systems, and business intelligence.🚀 Deployment-Ready ExpertiseWe are a team of drug development navigators and SMEs focused on accelerating clinical deployment.
Our Strengths
Outsourcing / Project Management & Global Monitoring – by Board Certified Multidisciplinary Subject Matter Experts
Product Development & Registration Strategies
Scientific & Medical Writing by Qualified Experts
Nonclinical & Clinical Strategy
Bioanalytical / Analytical / Immunology
Chemistry & Manufacturing
Investigator’s Brochures & Gap Analyses
Regulatory & Scientific Due Diligence
Investment Strategies (In-licensing, Technology Transfer, Dilutive/Non-Dilutive Funding)
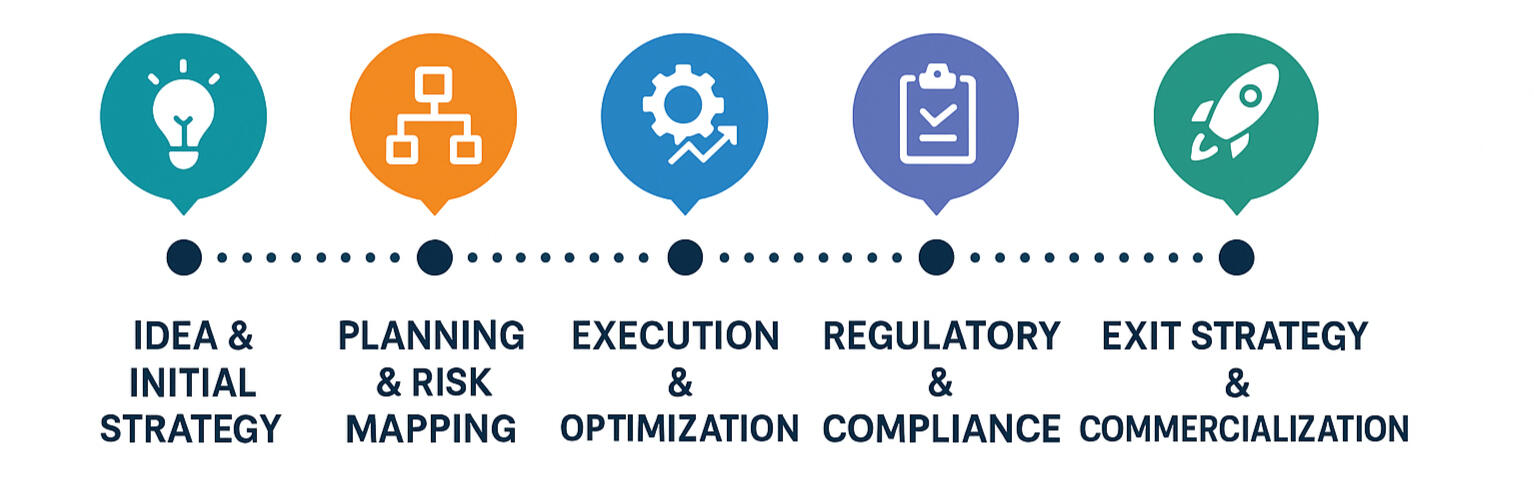
We design each trial with the end in mind—mapping critical paths, optimizing logistics, and ensuring timely delivery through smart, tailored strategies.
From Idea to Exit Strategy
The CCD Team Difference
CCD forms high-functioning, experienced teams tailored for each project. With board-certified SMEs and a track record of trial success, we deliver the quality and consistency our sponsors expect.Our team is comprised of oriented drug development professionals with a successful track record in trial outcomes.Our goal of creating the right project team for each trial ensures the consistency and quality sponsors expect.
What Sets Our Communication Apart
Built on Trust. Powered by Transparency.At CCD, we believe that effective communication starts with trust—and trust is earned. Our teams treat every project as the highest priority, ensuring clients feel confident and informed at every step.Proactive, Not Reactive.Our approach to project management is forward-thinking and meticulous. We anticipate challenges, streamline coordination, and keep studies on time and on budget—because great science deserves flawless execution.

Contact Us
Ready to optimize your clinical trial? Let’s build your path to market—faster, smarter, better.
Office:
1406-50 Absolute Avenue
Mississauga ON L4Z 0A8
Canada
Email:
[email protected]
Thank you
We will contact you shortly.